Instructions for use of the Myco Lid Pro®
AUTOCLAVING INSTRUCTIONS
- It is not recommended that the syringe filter be autoclaved by steam. They are OK to autoclave with dry heat. Although they are hydrophobic, steam still moves through the membrane and leaves the filter moist which may lead to decreased porosity of the filter and a reduction in diffusion.
- If autoclaving a liquid culture via steam, it is recommended to leave the syringe filter OFF during sterilization.
- Allow for the autoclave to return to room temperature before opening. Allow for the glass jars and substrate to cool down evenly.
- Open the Autoclave in sterile conditions (HEPA laminar flow hood recommended) and attach a syringe filter to the air port.
- To attach a syringe filter: With downward pressure, rotate the syringe filter 180° clockwise to secure the filter into place.
- * If autoclaving via steam, and you decide to leave the syringe filter ON, do not secure the locking band all the way. Leave 1/4 turn loose to prevent buildup of pressure. Allow for the autoclave to cool down completely before opening. Open in a sterile environment. Immediately secure the locking band to the jar.
- The Myco Lid Pro® can withstand up to 1500 accumulative minutes of steam autoclaving. Functionality of the luer taper fittings will not degrade after multiple sterilizations.
- Label the Myco Lid Pro® after autoclaving only. Sterilization will deteriorate stick-on labels and will also allow for the absorption of ink from “permanent” markers into the lid. Remove any writing with alcohol before autoclaving.
____________________________________________________________
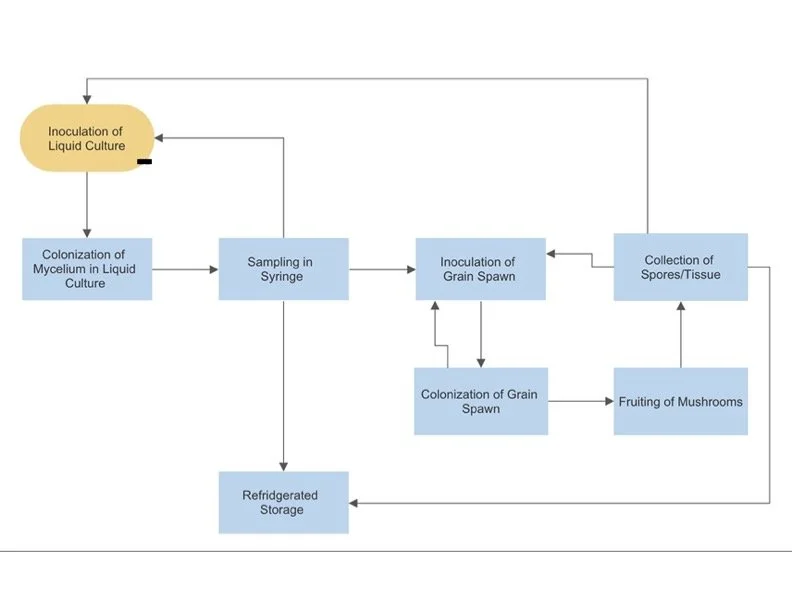
Liquid Culture Application
Step 1. Preparation
Create your sterilized liquid substrate in your chosen jar (methods vary). **Maintain sterile working conditions throughout handling.
• Materials Needed:
Myco Lid Pro®
Mason Jar (pint jar preferred for LC)
Locking Band
Self Healing Injection Port
Dispensing Needle (3” for pint jar)
Filter for chosen application
Luer Cap
Gasket
Culture Media
Stir Bar
*Stirring Media
Magnetic Stir Plate
Step 2. Inoculation
Inoculate the sterilized liquid substrate with hydrated spores or live culture using the self healing injection port. **Perform this step in sterile working conditions.
Step 3. Colonization of Liquid Substrate
Allow for the new mycelium/spores to begin taking over the liquid substrate. Once growth is observed, periodically mix with the magnetic stirrer every day to keep the mass from getting clumpy. Allow for the *stirring media to break apart the hyphae by vigorously blending the culture into a fluffy mass that then falls into a solid blanket at the bottom of the jar. This will take several weeks depending on the chosen species in culture. See demo videos.
Step 4. Sampling
Once the liquid substrate has been well colonized and a large mass of fluffy hyphae is in suspension, the culture is ready to sample.
**Prepare sterile working conditions
Prepare sterilized syringes and luer tips for sampling.
Place Liquid Culture on a magnetic stirring plate and stir the hyphae into suspension. this will allow for each sample to be consistent and homogenous.
Remove the luer cap on top of the Myco Lid Pro® and secure a sterile syringe to the luer tapered fitting.
Draw a sample from the jar, repeat as needed, seal syringes with sterilized luer caps. (pint jar can make 20+ Syringes) See sample videos.
Disinfect filled syringes and refrigerate for proper storage of samples.
If Prepared, (using a large sterile syringe) draw sterilized LC substrate from a reserve and refill the now drained LC jar using the sampling or injection ports in order to extend the life of the culture.
*Stirring Media for the demonstrated application are multiple plastic Luer Tapered Syringe Tips. The shape of the luer tip provides sharp edges to aid in breaking apart hyphae as the culture is stirred.
**Sterile working conditions are required to prevent contamination of the culture. For best results, use in conjunction with a HEPA Laminar Flow Hood.
Grain Spawn Application
Step 1. Preparation
Prepare your sterilized substrate in your Myco Lid Pro® and jar(s).
AUTOCLAVING INSTRUCTIONS
- It is not recommended that the syringe filter be autoclaved by steam. They are OK to autoclave with dry heat. Although they are hydrophobic, steam still moves through the membrane and leaves the filter moist which may lead to decreased porosity of the filter and a reduction in diffusion.
- If autoclaving via steam, it is recommended to leave the syringe filter OFF during sterilization.
- Allow for the autoclave to return to room temperature before opening. Allow for the glass jars and substrate to cool down evenly.
- Open the Autoclave in sterile conditions (HEPA laminar flow hood recommended) and attach a syringe filter to the air port.
- To attach a syringe filter: With downward pressure, rotate the syringe filter 180° clockwise to secure the filter into place.
- * If autoclaving via steam, and you decide to leave the syringe filter ON, do not secure the locking band all the way. Leave 1/4 turn loose to prevent buildup of pressure. Allow for the autoclave to cool down completely before opening. Open in a sterile environment. Immediately secure the locking band to the jar.
- The Myco Lid Pro® can withstand up to 1500 accumulative minutes of steam autoclaving. Some slight depression in the center of the lid may be observed after autoclaving and is normal. Functionality of the luer taper fitting will not degrade after multiple sterilizations.
- Label the Myco Lid Pro® after autoclaving only. Sterilization will deteriorate stick-on labels and will also allow for the absorption of ink from “permanent” markers into the lid. Remove any writing with alcohol before autoclaving.
• Materials Needed
Myco Lid Pro®
Mason Jar(s) and Locking Band(s)
Self Healing Injection Ports
Filter
Gasket
Substrate (I.e. Rye Berries)
Step 2. Colonization of Grain Spawn
With the samples taken from spores or liquid culture, Inoculate the sterilized mason jars using the self healing injection ports. ***Perform this step in sterile working conditions.
Allow for colonization to begin.
If desired, shake jars to break up the partially colonized substrate in an effort to spread the mycelium evenly around the jar. This may assist in faster colonization of the substrate.
—> Note: The air port design under the Myco Lid Pro® is integrally screened to keep grain from coming in contact with the filter material when shaken.
Once fully colonized, the substrate is ready for fruiting and can be removed from the jar.
Full colonization takes several weeks depending on the chosen species.
***Sterile working conditions are required to prevent contamination of the grain spawn. For best results, use in conjunction with a HEPA Laminar Flow Hood.